Propane: A Versatile Fuel with Expansive Utility
Propane, a hydrocarbon with the chemical formula C3H8, is a remarkable fuel known for its versatility and adaptability. It serves as a valuable energy source in various forms, and its applications are as diverse as its properties. In this expanded article, we will take a deeper look into what propane is, explore its chemical and physical properties, understand its production methods, delve into its myriad uses, and emphasize the paramount importance of handling it safely.
what is Propane?
Propane is a hydrocarbon gas that can transition into a liquid state under specific temperature and pressure conditions. It is distinguished by its odorless, colorless, and tasteless nature, making it challenging to detect without specialized equipment.
What’s in Propane?
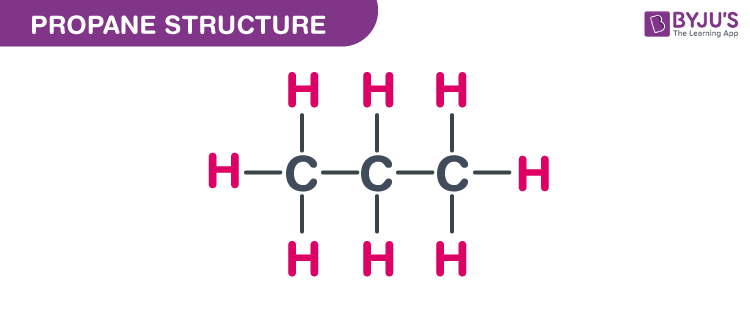
Propane is a hydrocarbon gas composed of molecules containing three carbon atoms and eight hydrogen atoms, represented by the chemical formula C3H8. It is odorless, colorless, and tasteless in its natural state.
what are the Chemical and Physical Properties of Propane?
Propane’s chemical and physical properties are key to its widespread applications. These properties include:
- Chemical Formula: C3H8
- Molecular Weight: 44.10 g/mol
- Physical State: Propane can exist as a gas or a liquid, depending on temperature and pressure.
- Boiling Point: -42.2 °C
- Melting Point: -187.6 °C
- Color: Odorless and colorless
- Taste: Odorless and tasteless
- Density: 0.504 kg/m3
what are the Chemical Reactions of Propane?
Propane, like other alkanes, undergoes combustion reactions in a similar fashion. When exposed to an excess of oxygen, propane combusts, yielding carbon dioxide and water.
The combustion reaction is represented as:
C3H8 + 5O2 → 3CO2 + 4H2O + heat
However, when there is an insufficient or excessive supply of oxygen, incomplete combustion occurs, resulting in the formation of soot (carbon) and/or carbon monoxide.
Incomplete combustion reactions can be exemplified by:
- 2C3H8 + 9O2 → 4CO2 + 2CO + 8H2O + heat
- C3H8 + 2O2 → 3C + 4H2O + heat
Propane is known for its high hydrogen content, making it burn hotter compared to substances like diesel fuel or home heating oil. The presence of C–C bonds in propane contributes to the distinct flame it produces during combustion.
how Propane is produced?
The production of propane primarily involves its extraction from two primary sources: natural gas and petroleum. Among these, natural gas stands as the more prevalent source of propane. During the refining process of these fossil fuels, propane is separated and collected as a valuable byproduct.
what are the Uses of Propane?
Propane’s adaptability is showcased in its diverse range of applications, making it a vital energy source in various sectors:
- Fuel for Heating: Propane plays a pivotal role in heating applications, whether it’s in homes, businesses, or industrial settings. It fuels furnaces, space heaters, and even ornamental fireplaces, providing a reliable source of warmth.
- Cooking: In areas where natural gas pipelines are not readily available, propane is a go-to choice for stovetops and ovens. It offers precise temperature control, making it a favorite for professional and home chefs alike.
- Grilling: Propane’s convenience and consistent heat have made propane-powered grills a ubiquitous feature in outdoor cooking and barbecues.
- Vehicle Fuel: As an alternative fuel, propane is used in vehicles, particularly in fleet operations such as buses and delivery trucks. Its lower emissions and cost-effectiveness make it an environmentally friendly choice.
- Refrigerant: Propane’s properties are utilized in small domestic refrigeration systems. It has also gained attention as an environmentally responsible replacement for certain ozone-depleting and greenhouse gas refrigerants.
- Feedstock for Petrochemicals: Beyond its role as a fuel, propane serves as a critical raw material in the production of various petrochemicals. It contributes to the manufacturing of plastics, synthetic rubber, and other essential products in the petrochemical industry.
what are the Disadvantages of Propane?
Propane, while versatile, is not without its drawbacks:
- Flammability: Propane is highly flammable, which can be a safety concern if not handled properly. Accidental leaks or mishandling can lead to fires or explosions.
- Storage and Transportation: Storing and transporting propane can be challenging due to its high pressure when in a gaseous state. Specialized containers and safety precautions are necessary.
- Environmental Impact: While considered cleaner than some other fossil fuels, burning propane still releases carbon dioxide into the atmosphere, contributing to greenhouse gas emissions.
- Limited Availability: In some regions, the availability of propane can be limited, making it less practical for certain applications or in emergency situations.
what is The Difference Between Propane and LP Gas?
The terms “propane” and “LP gas” are often used interchangeably, but there’s a subtle difference:
- Propane: This is the chemical name of the gas and refers to the specific hydrocarbon compound C3H8.
- LP Gas: LP stands for “liquefied petroleum gas,” which is a broader term encompassing various gases, including propane, butane, and isobutane. Propane is the most common component of LP gas, making it the primary gas in LP gas blends.
Why Propane Is Preferred Over Natural Gas?
Propane offers several advantages over natural gas:
- Portability: Propane is stored in tanks, making it highly portable. This is particularly useful in outdoor settings, like for grilling or in recreational vehicles.
- Energy Density: Propane has a higher energy density compared to natural gas, which means it can produce more heat for the same volume.
- Versatility: Propane can be used in areas where natural gas pipelines are not available. It’s a reliable choice for heating, cooking, and powering vehicles in such locations.
- Clean-Burning: While not entirely emissions-free, propane produces fewer emissions, including carbon monoxide and nitrogen oxides, compared to some other fossil fuels, contributing to better air quality.
- Storage and Shelf Life: Propane can be stored for long periods without deterioration, making it a dependable fuel source, even during emergencies or power outages.
In summary, propane is not without its disadvantages, such as flammability and environmental concerns. However, it is a valuable and versatile energy source. It differs from natural gas, with advantages including portability, higher energy density, and the ability to be used in areas without natural gas infrastructure. Its cleaner-burning nature and long shelf life also make propane a preferred choice for various applications.
how is Propane in Dryers?
Propane-powered dryers, sometimes referred to as propane gas dryers, operate by using propane as the primary fuel source to generate heat. can gas dryer run on propane, This heat is then used to evaporate moisture from clothing, effectively drying them. Check if can you use a gas dryer without gas
how does a gas dryer work ? Propane dryers have a few key components:
- Propane Burner: The propane burner is responsible for heating the air inside the dryer. It ignites when the dryer is in use, producing the heat necessary for the drying process.
- Heat Exchanger: This component allows the hot air from the propane burner to transfer its heat to the circulating air in the dryer drum.
- Thermostat: To control the drying process, propane dryers are equipped with a thermostat that helps maintain the desired temperature inside the dryer thermal fuse keeps blowing sometimes.
what are the Advantages of Propane-Powered Dryers?
Propane dryers offer several advantages over their electric counterparts, it might be a problem if gas dryer smells like gas but still making them a preferred choice for many homeowners:
- Efficiency: Propane dryers are known for their efficiency in drying clothes quickly and effectively. The heat produced by propane is often hotter than electric dryers, which means faster drying times and potentially lower energy costs. You may ask if Can You Convert Electric Dryer to Gas.
- Cost-Effective: Propane is often more cost-effective than electricity for drying large loads of laundry. This can result in energy savings over time, especially in areas where propane is readily available and affordable.
- Environmental Impact: While not entirely emissions-free, propane is considered a cleaner-burning fuel compared to coal or oil. This can lead to reduced greenhouse gas emissions associated with the drying process. Read about the gas dryer outlet which is the source.
- Versatility: Propane dryers are not dependent on the availability of electrical outlets or the need for special wiring, making them suitable for use in various settings, including remote areas or homes where electricity is unreliable.
- Consistency: Propane dryers offer consistent heat, resulting in more uniform and efficient drying of clothes. This can help reduce wear and tear on garments compared to electric dryers that may have temperature fluctuations.
how to Prioritize Safety in Propane Use?
Given its flammable nature, safety is paramount when handling propane. It’s crucial to adhere to safety guidelines and recommendations provided by regulatory authorities. This includes proper storage, handling, and maintenance of propane tanks and equipment. Additionally, regular inspections and maintenance ensure that propane systems remain in good working order, reducing the risk of accidents.
conclusion
In conclusion, propane is indeed a remarkable and versatile fuel, seamlessly fitting into numerous aspects of our lives, from providing heat and powering cooking appliances to serving as an eco-friendly vehicle fuel and a foundation for the petrochemical industry. However, it should be emphasized that the safe use and handling of propane are non-negotiable. By understanding its properties and adhering to safety protocols, we can harness the benefits of propane while minimizing associated risks. Whether in our homes or industries, propane continues to be an invaluable and adaptable energy source.







